Our clinical trials
Cantargia’s clinical program
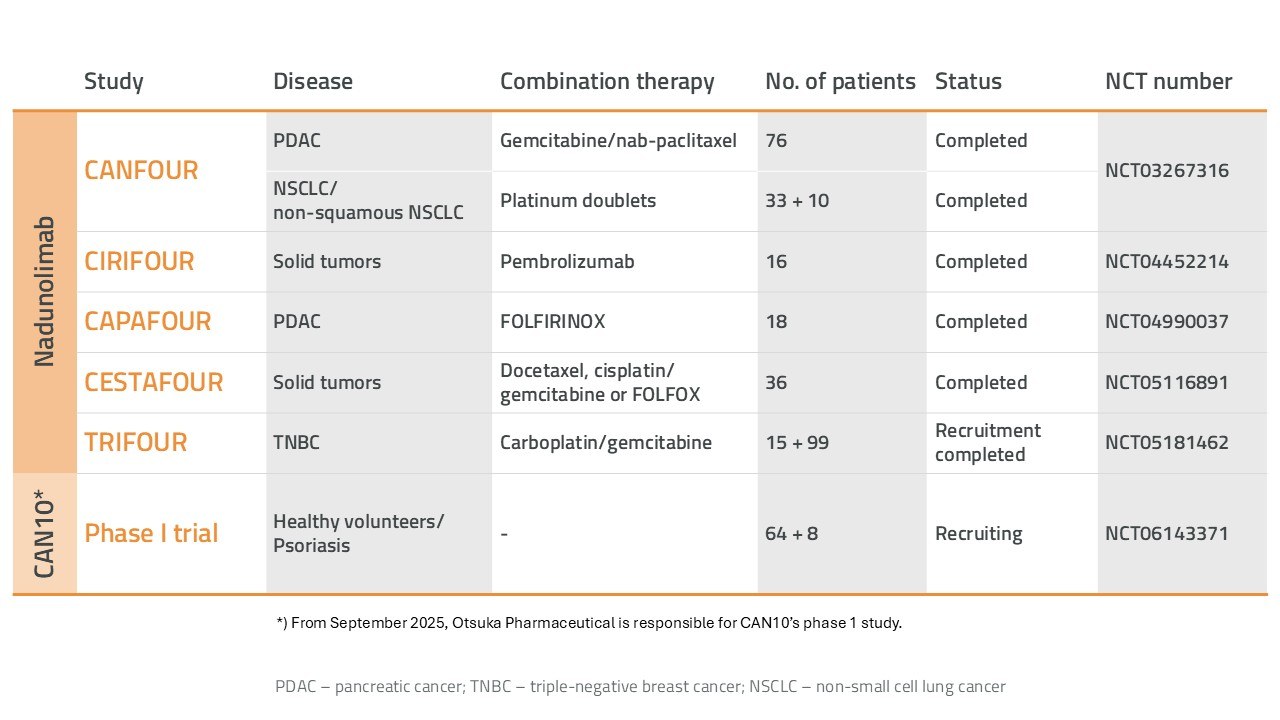
Overview of Cantargia’s clinical studies.
Nadunolimab (NADUNOLIMAB)
The IL1RAP-binding antibody nadunolimab (CAN04) is Cantargia's most advanced project. Nadunolimab has been evaluated in several clinical studies involving over 300 cancer patients, in combination with chemotherapy or immunotherapy, as summarized in the table above. The highest priority for the program is to develop a treatment for patients with pancreatic ductal adenocarcinoma (PDAC).
CAN10
The CAN10 project is developing an anti-IL1RAP antibody designed for the treatment of inflammatory and autoimmune diseases. The CAN10 antibody binds with high affinity to IL1RAP and blocks the function of signaling molecules IL-1, IL-33, and IL-36, all of which play a crucial role in various autoimmune and inflammatory diseases. In September 2023, a clinical Phase I study for CAN10 was initiated involving healthy volunteers and psoriasis patients. In 2025, Otsuka Pharmaceutical Co., Ltd. acquired the CAN10 program from Cantargia and will complete the ongoing Phase I study.

