Scientific concept
An antibody with multiple opportunities
Cancer is a collective term for over 200 different diseases where changes in genetic information has resulted in uncontrolled cell division. Tumors comprise many types of cells in addition to the cancer cells. These include various immune cells and so-called stromal cells, but also cells forming the blood vessels. A complex communication between cancer cells and these tumor-supporting cells contributes to tumor progression and growth.
Interleukin-1 in the tumor microenvironment
IL1RAP (interleukin-1 receptor accessory protein), the target of Cantargia’s most advanced asset CAN04 (nadunolimab), is found not only on cancer cells, but also on several of the tumor-supporting cell types within the tumor. In this context, IL1RAP is involved in transmitting signals from the two interleukin-1 (IL-1) molecules, interleukin-1 alpha (IL-1α) and interleukin-1 beta (IL-1β), which both contribute to a tumor microenvironment that favors tumor development and growth. For example, these signals can strengthen the tumor’s defences against immune cells that can attack and kill the tumor, or stimulate blood vessel formation in the tumor.
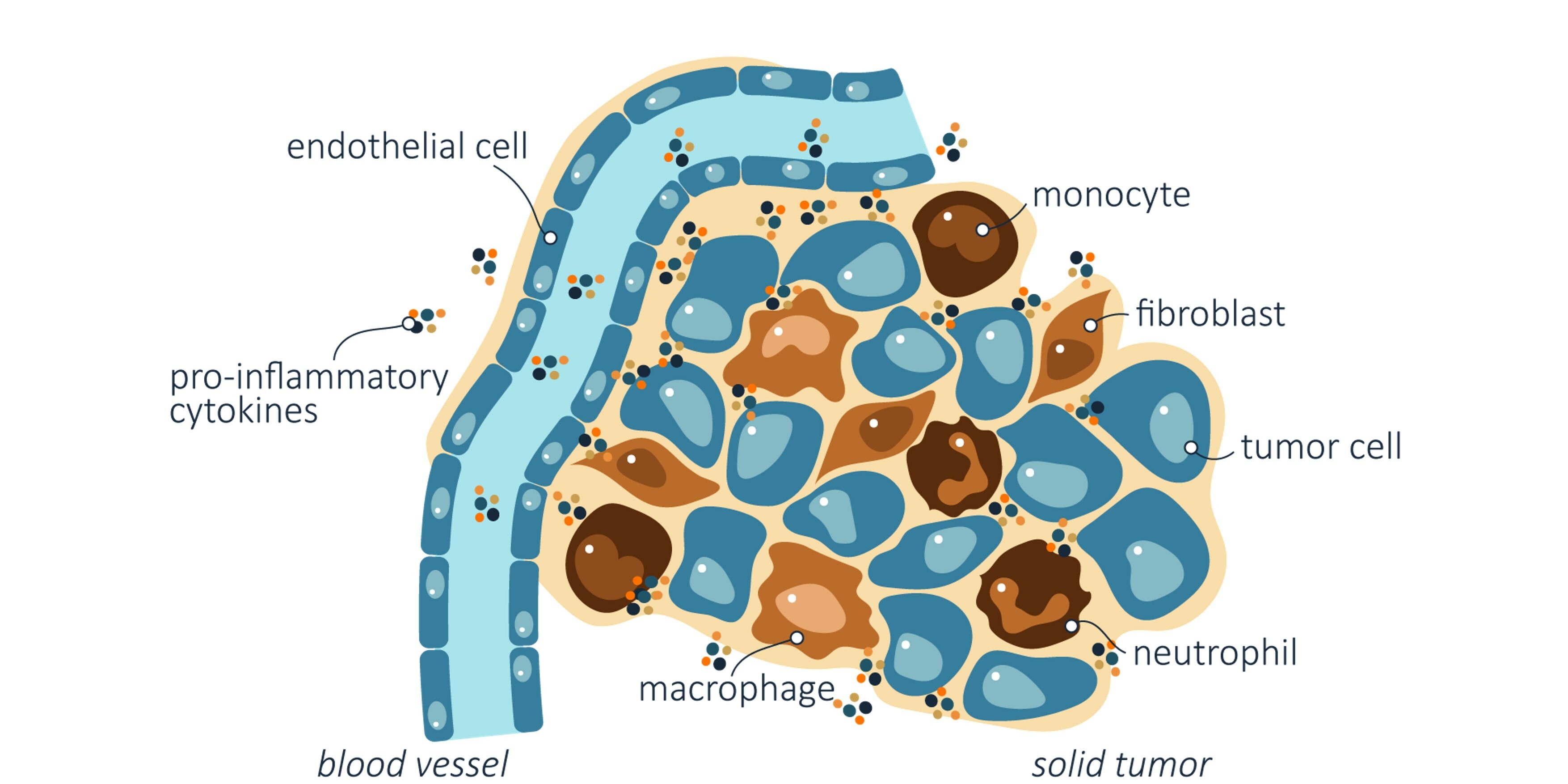
A tumor consists of cancer cells and a variety of tumor-supporting cells. Communication between these cells is mediated by different signals, so-called cytokines, including the IL-1 molecules.
CAN04 is unique in that it has a dual mechanism of action. By binding IL1RAP, CAN04 stimulates the so-called Natural Killer cells of the immune system to seek out and kill the cancer cells by a mechanism known as Antibody-Dependent Cellular Cytotoxicity (ADCC). It also blocks the tumor-promoting signaling of both IL-1α and IL-1β.
CAN04 synergizes with chemotherapy
Cytostatics, or chemotherapy, are established standard treatments in a variety of cancer types and have been designed to kill rapidly dividing cells. However, these drugs rarely cure the disease and may cause severe side effects as they also affect healthy cells that divide rapidly. Another important function of CAN04 is its ability to potentiate the effect of chemotherapy drugs.
Previous research, as well as Cantargia’s own studies, have shown that treating cancer cells with chemotherapy triggers the cancer cells to release IL-1α [1]. This in turn stimulates the release of IL-1β from surrounding cells in the tumor. The presence of both IL-1α and IL-1β in the tumor increases the tumor’s ability to develop resistance to chemotherapy. As CAN04 blocks the signalling of both forms of IL-1, it is very well-suited for combination with chemotherapy.
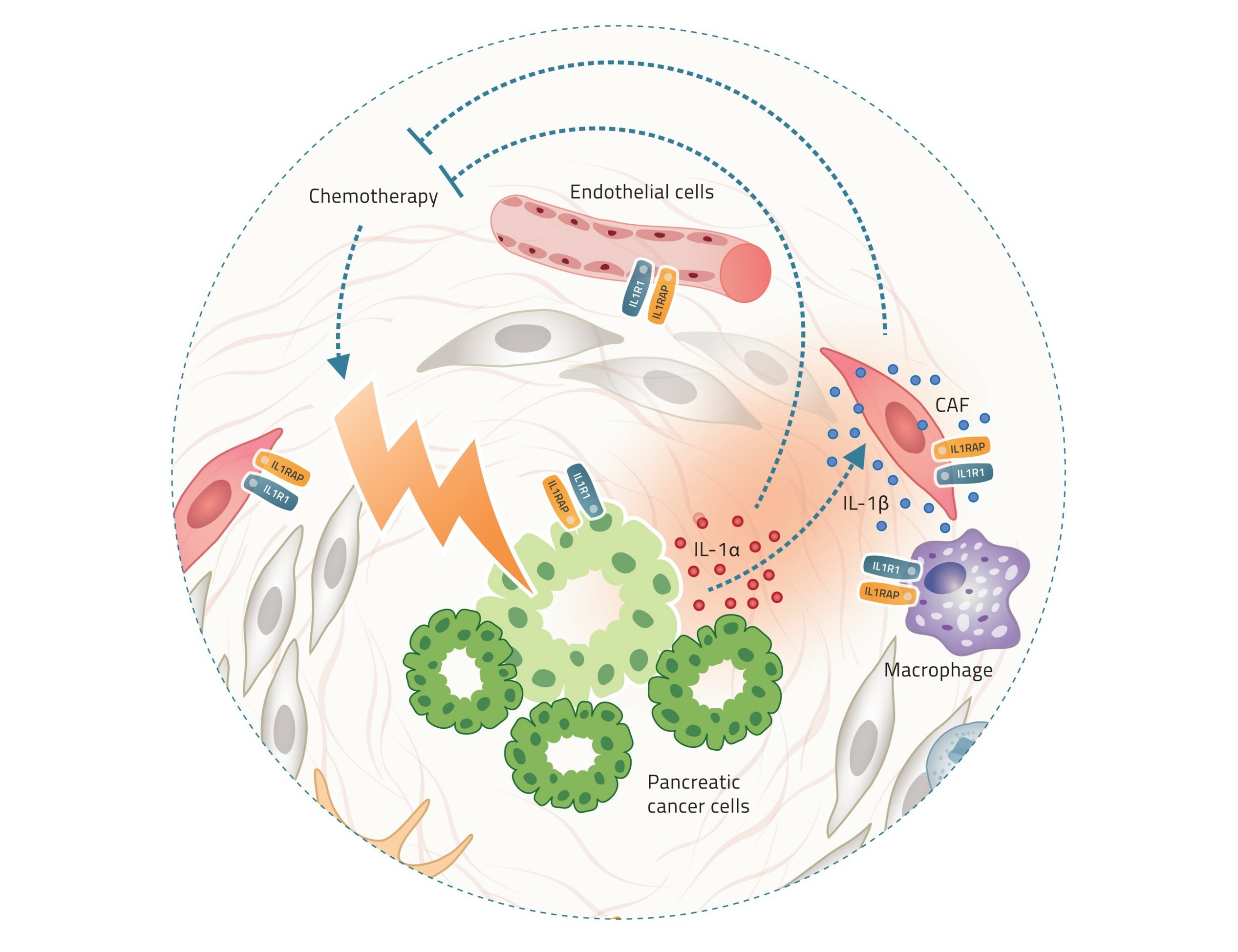
In the tumor, chemotherapy triggers the release of IL-1α by cancer cells, which in turn stimulates the release of IL-1β by stromal cells. Both IL-1α and IL-1β contribute to the tumor’s resistance to chemotherapy.
Preclinical studies have shown that CAN04 has very good antitumor efficacy in combination with chemotherapy. When CAN04 was combined with platinum-based chemotherapy, an antitumor effect was achieved that was much stronger than the effect of the individual therapies [1]. Preliminary clinical data point to similar effects in cancer patients; in over 70 pancreatic cancer patients and 30 non-small cell lung cancer patients evaluated in the phase I/IIa CANFOUR trial, CAN04 in combination with chemotherapy results in higher efficacy than historical controls for chemotherapy alone [2][3].
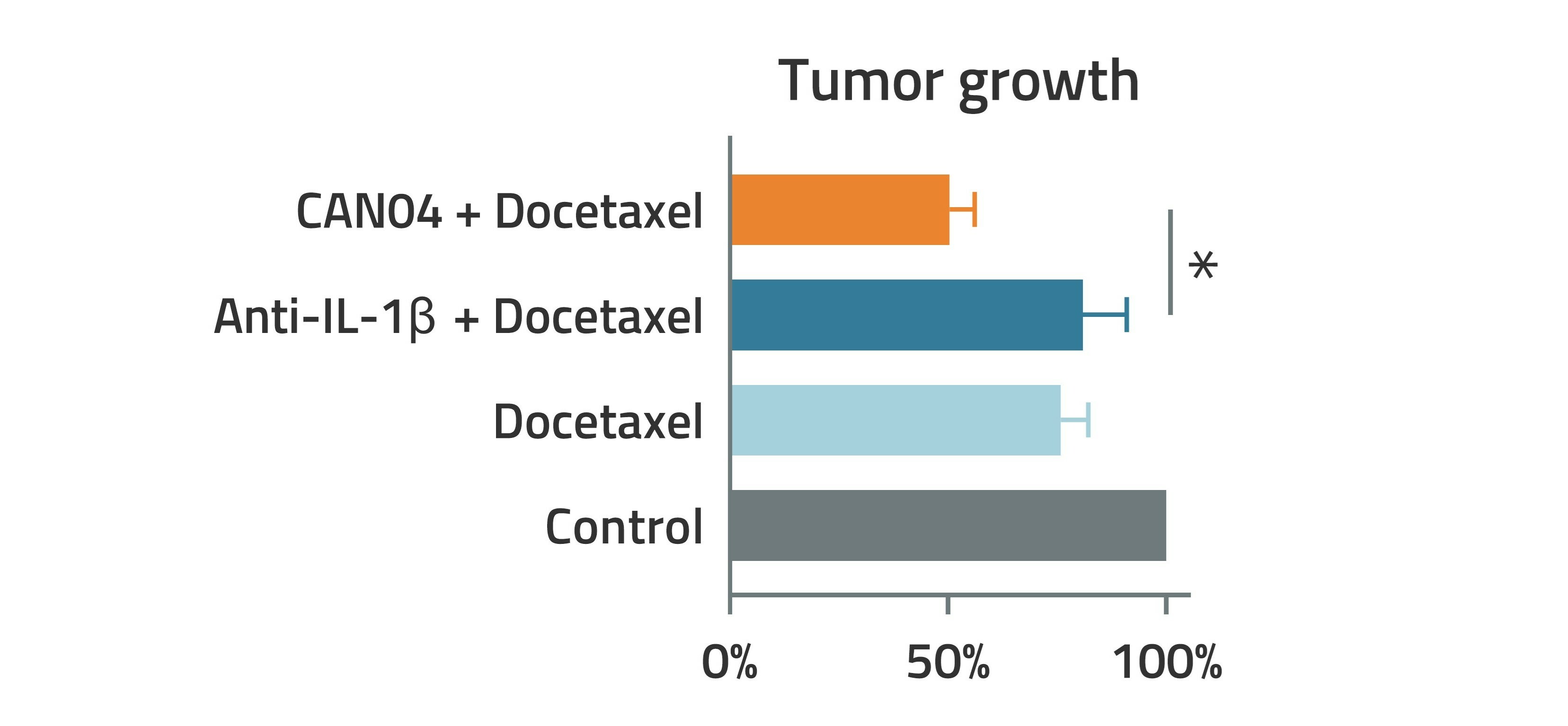
Preclinical studies show stronger antitumor effect of CAN04 in combination with the chemotherapy docetaxel, compared to docetaxel in combination with an antibody which only blocks IL-1β.
Preclinical data also showed that the combination of CAN04 and the chemotherapy docetaxel produces a stronger antitumor effect than docetaxel alone, or docetaxel combined with an antibody that blocks only IL-1β [1]. This strengthens the hypothesis that a broader effect on the IL-1 system, which is achieved when CAN04 binds to IL1RAP, is necessary to reduce the tumor’s resistance to chemotherapy. Blocking only one form of IL-1 is thus not sufficient to achieve this effect.
References
[1] Rydberg Millrud et al, Cancer Immunol Immunother 2022; Epub ahead of print
[2] Paulus et al, J Clin Oncol 2022; 40:9020
[3] Van Cutsem et al, J Clin Oncol 2022; 40:4141

