CAN10
CAN10 – Anti-IL1RAP antibody for treatment of autoimmune and inflammatory diseases
CAN10
In September 2025, Otsuka Pharmaceuticals' acquisition of the CAN10 program was completed. CAN10 is reported as part of Cantargia's portfolio of strategic partnerships.
CAN10 is an IL1RAP-directed antibody that has a unique ability to block signaling not only from IL-1, but also from IL-33 and IL-36. Simultaneous blocking of all three of these cytokines has great potential for the treatment of multiple, often heterogeneous autoimmune and inflammatory diseases.
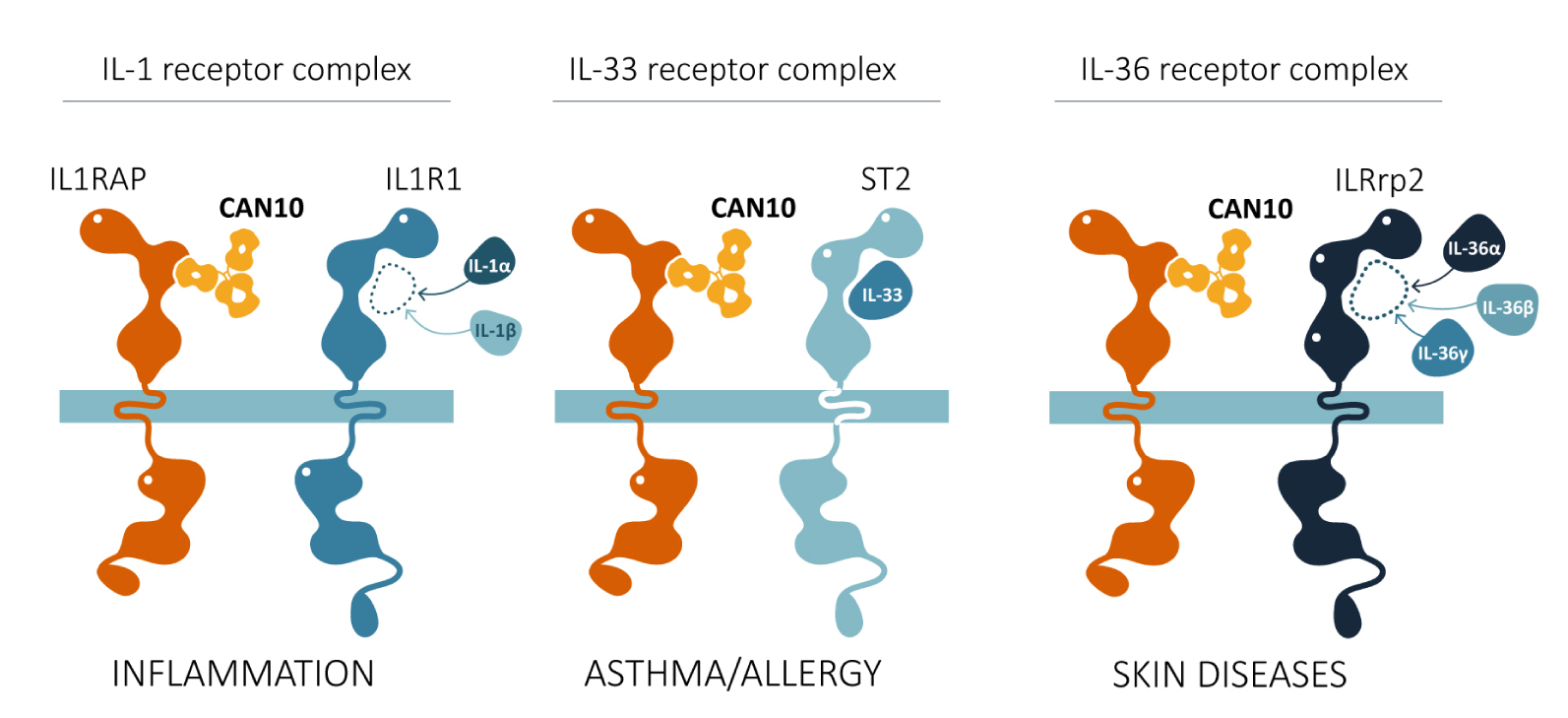
The CAN10 antibody blocks IL-1, IL-33, and IL-36.
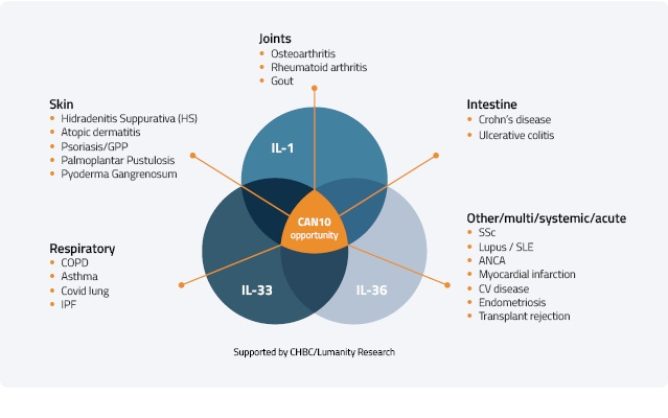
The applicability of using CAN10 in various immunological diseases is shown in the figure below.
CAN10 is also developed to minimize binding to effector cells. This means that CAN10, unlike nadunolimab (CAN04), does not stimulate cell killing via Antibody-Dependent Cellular Cytotoxicity (ADCC).
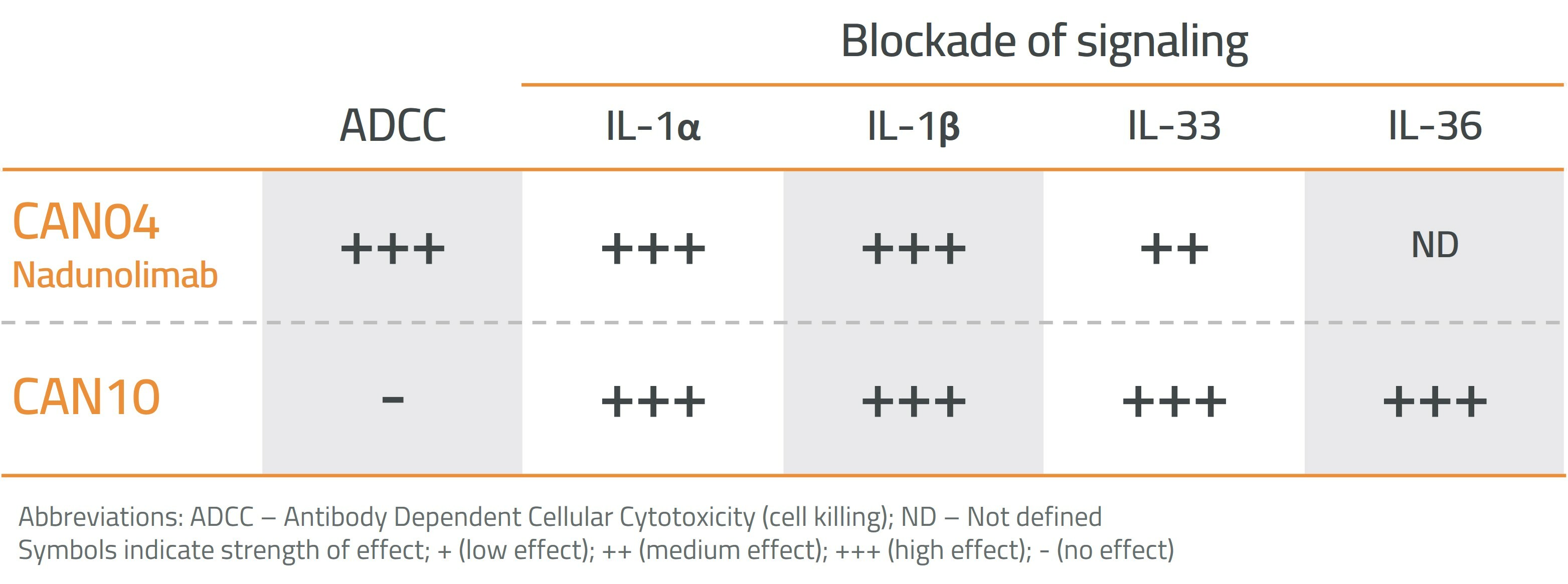
CAN04 and CAN10 have different functional properties, where CAN04 is developed for the treatment of cancer and CAN10 for the treatment of autoimmune and inflammatory diseases.
Clinical development
The first Phase 1 clinical study (NCT06143371) with CAN10 is nearing completion. Treatment of healthy volunteers has been completed and the part with participants with psoriasis are continuing. Results from the study have been continuously reported to the market and no safety concerns have been observed at the different dose levels tested so far. In addition, very promising and strong biomarker data have been reported, indicating prolonged biological activity and possibilities for dosing every 4 weeks.

