Cantargia reports high response rates with CAN04 combination therapy in NSCLC and presents next development steps
Cantargia AB today announced updated interim results from the ongoing clinical trial investigating the interleukin-1 receptor accessory protein (IL1RAP) blocking antibody CAN04 in combination with chemotherapy in non-small cell lung cancer (NSCLC). Tumor burden decreased in all 9 evaluable patients and in 6 of these patients, the decrease is large enough to be defined as a response. One patient treated for more than one year has an ongoing complete response and the other five have partial responses. The side effects are those expected from chemotherapy or CAN04. Neutropenia is more frequent than anticipated from chemotherapy alone, a manageable side effect known to be associated with IL-1 pathway blockade. Due to disruptions from the COVID-19 pandemic and changing treatment standards in NSCLC, recruitment is expected to be slower than planned during Q4 2020. As the necessary efficacy information for next step development during 2021 already is in place, less than the planned 31 patients may be included in this study.
Cantargia develops antibody-based pharmaceuticals against IL1RAP. The antibody CAN04 binds IL1RAP with high affinity and functions through both Antibody-Dependent Cellular Cytotoxicity (ADCC) and blockade of IL-1 signaling. Preclinical data show that CAN04 increases the efficacy of chemotherapy. CAN04 is investigated in a phase I/IIa clinical trial, CANFOUR, examining combination with two different, frequently used chemotherapy regimes in patients with NSCLC or pancreatic cancer (PDAC) in first line chemotherapy setting (https://clinicaltrials.gov/ct2/show/NCT03267316). Early interim combination therapy data showing higher than expected response rates compared to chemotherapy alone was presented in December 2019. Recruitment in the PDAC arm is following communicated timelines with results expected during Q4 2020.
In the NSCLC combination cohort, an updated interim analysis has been performed. Thirteen patients have started therapy with 5 mg/kg CAN04 in combination with the platinum doublet gemcitabine/cisplatin, nine patients have been treated for more than two months and can be evaluated for efficacy. Two patients started treatment less than 2 months ago and can therefore not yet be evaluated. Two patients have been censored due to early consent withdrawal unrelated to their cancer. All 9 evaluated patients had a decrease in tumor burden during therapy and, so far, 6 of these have reached a response according to the current standard criteria (RECIST 1.1, more than 30 % decrease in tumor diameter), including one patient with an ongoing complete response (CR) that has been durable for 12 months of treatment and 5 patients with partial response (PR). The remaining 3 patients have stable disease and no patient scored progressive disease at the 2-month analysis. This response level is much higher than expected with the platinum doublet alone, which has shown a response rate of 22-28 % in first line patients1,2. Of the evaluated patients, 5 have previously received immunotherapy before entering this trial, while 4 have not received previous therapy. Below is a summary of the response data in a so-called waterfall plot showing the best change in tumor burden for the evaluable patients so far.
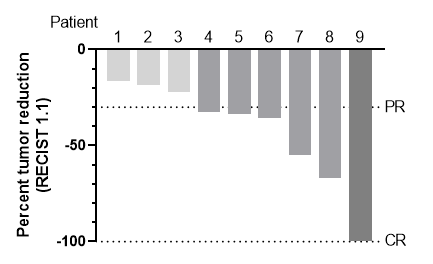
The side effects are those expected from the gemcitabine/cisplatin combination or CAN04. Neutropenia incidence has, however, been higher than expected from chemotherapy alone. Neutropenia is a side effect coupled to both chemotherapy and IL-1 blockade and can be managed by e.g. dose reductions or standard treatment with growth factors.
Recruitment to the NSCLC arm of the CANFOUR trial is slower than expected and due to an increase of COVID-19 in some key territories, recruitment rate during Q4 will not increase as planned, despite the opening of new centers. In addition, chemotherapy using platinum doublets are more and more reserved for first line combination with pembrolizumab, decreasing the patient population available for the ongoing trial. As Cantargia already have generated enough data to advance the development of CAN04 in NSCLC, the following strategy will be implemented:
-
To generate more data, additional patients will be allowed to enter the trial during 2020 but there is no longer a need to reach the 31 planned patients. To obtain more information around doses, lower dose levels will be investigated to potentially optimize treatment and also receive initial results to describe relationships between dose and pharmacological effects.
- Start preparations for development of CAN04 into first line therapy together with immunotherapy and platinum doublet as well as second line therapy in combination with docetaxel during 2021. These are major NSCLC segments today. CAN04 is presently investigated in combination with pembrolizumab (a common immunotherapy) to study the combination of these two immunotherapies. The results in this trial together with those generated in CANFOUR can be combined for effective development.
“We are excited over the new interim efficacy results, confirming the high response rate previously observed for this combination. It is therefore logical to advance the development of CAN04 in NSCLC for use in larger groups of patients”, says Göran Forsberg, CEO of Cantargia. “In addition to our ongoing CAN04 development in pancreatic cancer, next step activities in NSCLC is currently being prepared for initiation during 2021”.
References
1 Schiller et al, N Engl J Med 2002; 346: 92–98
2 Scagliotti et al, J Clin Oncol 2008; 26: 3543–3551
For further information, please contact
Göran Forsberg, CEO
Telephone: +46 (0)46-275 62 60
E-mail: goran.forsberg@cantargia.com
The information was submitted for publication at 12.15.00 CET on 23 September 2020.
About Cantargia
Cantargia AB (publ), reg. no. 556791-6019, is a biotechnology company that develops antibody-based treatments for life-threatening diseases. The basis for this is the protein IL1RAP that is involved in a number of diseases and where Cantargia has established a platform. The main project, the antibody CAN04, is being studied in the clinical phase I/IIa CANFOUR study with a primary focus on non-small cell lung cancer and pancreatic cancer. The study is focused on combination therapies, but also includes a monotherapy arm. Positive interim data from the combination therapies were presented in December 2019. Cantargia’s second project, the antibody CAN10, addresses treatment of serious autoimmune/inflammatory diseases, with initial focus on systemic sclerosis and myocarditis.
Cantargia is listed on Nasdaq Stockholm (ticker: CANTA). More information about Cantargia is available at http://www.cantargia.com.


